The Ethical Quagmire of Medical Trials
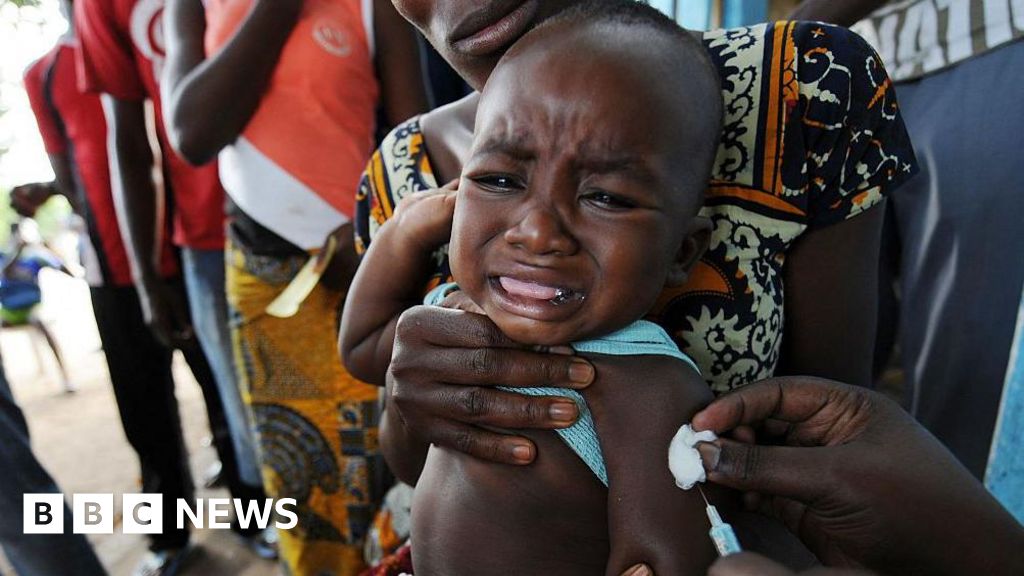
The ongoing debate surrounding a $1.6 million baby vaccine trial in Guinea-Bissau transcends mere public health discussion; it raises fundamental ethical questions that resonate across global health frameworks. Billed as an investigation into the hepatitis B vaccine's broad health impacts, the trial sought to administer the vaccine at birth to one cohort while delaying treatment for another. The World Health Organization (WHO) has squashed these plans, branding them 'unethical' due to the potential harm posed to infants deprived of a vaccine proven to be vital in preventing transmission.
WHO's Stance and Public Health Guidelines
The WHO insists on immediate vaccination — a crucial intervention globally recognized as a public health necessity. It notes that vaccinating newborns at birth can prevent hepatitis B transmission from mother to child in 70-95% of cases. The organization's concern extends to the scientific basis of the study, citing a lack of compelling justification for withholding a life-saving vaccine from a set of vulnerable newborns.
"We have significant concerns about the scientific justification, ethical safeguards, and alignment with established standards for human research," said a WHO representative.
The Reaction from the Guinea-Bissau Public
Public outcry precipitated a government intervention, halting the trial just months before it was slated to commence. Prominent figures, including former health minister Magda Robalo, have vocally condemned the study, insisting that citizens should not be subjected to such compromised health trials. "Guinea-Bissauans are not guinea pigs," Robalo emphasized in interviews with major media outlets, echoing sentiments that challenge the ethical structures surrounding vaccine trials in global health settings.
The Broader Implications
At its core, this controversy underscores a more significant issue: the ethical implications of public health initiatives in low-income nations versus developed ones. Some critics argue that experimental trials often target less affluent nations due to perceived weaker regulatory environments, abandoning the ethical rigor applied in wealthier countries. The implications are profound. With 12% of Guinea-Bissau's adult population reportedly living with chronic hepatitis B, maintaining ethical integrity in vaccine administration is paramount.
The Challenges in Global Health
The U.S. Department of Health, under Robert F. Kennedy Jr., sought to study the vaccine's wider health repercussions. However, Kennedy's prior comments and actions questioning vaccine efficacy have led to skepticism and distrust both locally and globally. It raises the question: Should experimental trials be conducted in locations where scientific consensus about treatment protocols is lacking?
Consent and Autonomy
The issue of informed consent is perfunctory yet pivotal in this discussion. The WHO's guidelines stipulate that any medical trial must have explicitly informed participants, ideally achieving a well-rounded understanding of the benefits and risks involved. In Guinea-Bissau, where healthcare access is routinely limited, ensuring comprehensive consent from families likely to be enrolled in the trial poses unique challenges.
Conclusion: Reflecting on Ethics in Medicine
The fallout from this vaccine trial not only questions the methodologies of public health initiatives but also serves as a stark reminder of our responsibility as global citizens to safeguard the dignity and well-being of vulnerable populations. By critically examining ethical considerations and the power dynamics at play in global health research, we may achieve more humane and equitable solutions that prioritize people over politics.
For Further Reading
Source reference: https://www.bbc.com/news/articles/c78j5k2gk02o




Comments
Sign in to leave a comment
Sign InLoading comments...